Abstract
Background. Azacitidine (AZA) and venetoclax (VEN) combination is approved in previously untreated acute myeloid leukemia (AML) patients ineligible to intensive chemotherapy (IC). However, AZA-VEN is associated with toxicities that often requires dose reduction (or discontinuation) and may limit triplet combination tolerance. In our study, we evaluated efficacy and toxicity of VEN exposure reduction by limiting VEN intake to the concurrent days of AZA administration (7+7 scheme).
Methods. We retrospectively analyzed data from untreated AML patients ineligible for IC who received AZA for 7 days associated with VEN for only 7 days from the 1st cycle every 28 days. Response to therapy was evaluated according to ELN-2022 criteria's and comparisons between cohorts were determined using Chi2 test. Time-to-event analysis were assessed using the log-rank method, with logistical regression analysis and Cox proportional hazard modeling to determine the effect of variables.
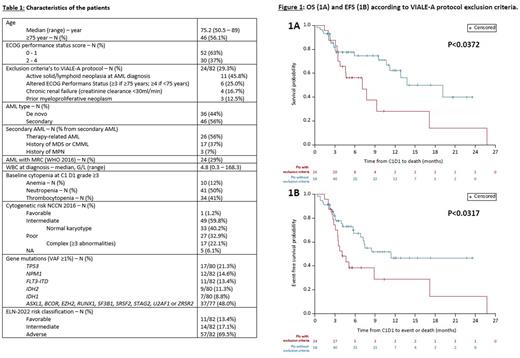
Results. Charts from 82 treatment-naïve AML patients from 7 french centers who received AZA-VEN 7+7 scheme from the 1st cycle were retrospectively analyzed (Table 1). Median age was 75.2 years and 56% had secondary AML (including 26 patients with therapy-related AML). Performance status (PS) was altered (i.e. PS 2-4) in 37% of patients and 29% presented at least 1 exclusion criteria to VIALE-A protocol. Forty percent of patients presented normal karyotype while 22% had complex cytogenetic. Myelodysplasia-related genes mutations were found in 48%, TP53 mutations in 21.3% of patients while NPM1 and IDH2 mutations were found in 14.6% and 13.4% respectively. Overall, 69.5% of patients presented adverse risk as defined by ELN-2022 classification.
Patients received a median of 4 cycles (range 1-28). After cycle 1, 49% presented febrile neutropenia grade III/IV and 88% required at least 1 transfusion. Early death rate at 60 days was 6.1%. Neutrophil (>1G/L) and platelet (>100G/L) recoveries were observed after a median of 36 and 31 days respectively. Forty-eight percent of patients required a median delay of 13 days to start the 2nd cycle. Overall response rate (ORR; complete remission (CR) and complete remission with incomplete hematologic recovery (CRi)) were 41.5%, 53.9% and 68.3% after 1, 2 and all cycles respectively. Univariate analysis revealed that complex karyotype, TP53 mutations (VAF≥10%) and adverse risk ELN-2022 were correlated with a worse ORR. With a median follow-up of 4.8 months (0.3-25.8), estimated event-free survival (EFS) and overall survival (OS) were 7.5 months (IC95: 5.1-17.1) and 12.8 months (IC95: 9.2-19.2) respectively. Univariate analysis revealed that OS and EFS were significantly improved in patients without VIALE-A protocol exclusion criteria's, ≥75 years, with PS 0-1, normal karyotype and in absence of TP53 mutation. Thus, estimated median EFS and OS in patients without VIALE-A protocol exclusion criteria's were 11.4 and 13.8 months respectively (Fig 1).
Among 56 CR/CRi patients, 61% had dose reduction mainly due to grade III/IV cytopenias: reduced treatment duration to 5 days in 97%, VEN dose reduction to 200mg/d in 50% and a 5-weeks interval between cycles in 29% of patients. Estimated median OS in patients with reduced dose was 25.8 months.
Discussion. Limiting VEN exposure to the concurrent days of AZA administration (7+7 scheme) induced similar response rates than the recommended VEN administration for 28 days. The 7+7 scheme, applied to the same population than VIALE-A trial, compared favorably to the 7+28 usual dose. Even with reduced VEN exposure, significant toxicity occurred during cycle 1 and subsequent cycles. In responders, subsequent dose reduction of VEN seems associated with favorable outcome. Our results provide rationale for reduced AZA-VEN scheme used in current clinical trial evaluating triplet combination.
Disclosures
Willekens:Abbvie: Honoraria. Bonnet:Abbvie: Honoraria. Micol:AstraZeneca: Honoraria; Abbvie: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Astellas: Honoraria. Marzac:Astellas: Honoraria. Jourdan:BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Bouscary:Abbvie: Honoraria. de Botton:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Honoraria, Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees; AURON: Research Funding; FORMA: Research Funding; SYNDAX: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Other: Travel / accommodation, Speakers Bureau; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel / accommodation, Speakers Bureau; Jazz pharmaceutical: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal